
/GettyImages-10181168-571578a73df78c3fa235c740.jpg)
Matter is the stuff all around us: your computer, the air you breathe, your lunch, even you are made up of matter. By understanding chemistry you can better understand the world around you and how it works. Even chefs use chemistry to cook delicious meals. Farmers use chemistry to help their crops to grow so we can have food.
ATOM DEFINITION CHEMISTRY TV
Engineers use chemistry to make electronics like your TV and your cell phone. chemistry synonyms, chemistry pronunciation, chemistry translation, English dictionary definition of chemistry. Doctors use chemistry to make medicine that helps us when we are sick. Scientists who specialize in chemistry are called chemists.Ĭhemistry is used all around us. A more precise definition might be that elements can be sub - divided into atoms. Sometimes chemistry is called the "central science" because it is an important part of other major sciences such as biology, Earth science, and physics. We now know that atoms can in fact be broken down into sub-atomic particles. Chemistry is considered a physical science and is closely related to physics. If water is a molecule, is it also a compound because the hydrogen and oxygen have been chemically combined? If so.Chemistry is the branch of science that studies the properties of matter and how matter interacts with energy. What is the difference between a compound and a molecule? Steve Gagnon, Science Education Specialist ( Other answers by Steve Gagnon) Some examples of mixtures are a tossed salad, salt water and a mixed bag of M&M's candy. A mixture can usually be separated back into its original components. Some examples of compounds are water (H 2O), table salt (NaCl), table sugar (C 12H 22O 11) and chalk (CaCO 3).Ī mixture is a substance made by combining two or more different materials in such a way that no chemical reaction occurs. All known elements are arranged on a chart called the Periodic Table of Elements.Ī compound is a substance made from two or more different elements that have been chemically joined. If you change the number of neutrons an atom has, you make an isotope of that element. We will present you the development of atom models and the chemical reactions related to atom.
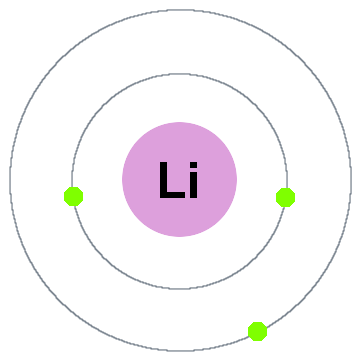
This article will cover the chemistry models of atoms. Knowing the basic concept of proton, electron, neutron is helpful to understand the chemistry models of atoms. If you change the number of protons an atom has, you change the type of element it is. Atom consists of three main parts which are proton, electron and neutron. All isotopes of a particular element have the same number of protons, but can have different numbers of neutrons. The number of protons, neutrons, and electrons in an atom can be determined from a set of.

These different versions of hydrogen are called isotopes. If you had very, very good eyes and could look at the atoms in a sample of hydrogen, you would notice that most of the atoms have no neutrons, some of them have one neutron and a few of them have two neutrons. For example, the element hydrogen is made from atoms containing just one proton and one electron. A particular atom will have the same number of protons and electrons and most atoms have at least as many neutrons as protons.Īn element is a substance that is made entirely from one type of atom. The protons and neutrons cluster together in the central part of the atom, called the nucleus, and the electrons 'orbit' the nucleus. What is the simplest way of explaining what atoms, elements, compounds and mixtures are?Ītoms are the smallest bits of ordinary matter and are made from particles called protons (which carry a positive electrical charge), neutrons (which carry no electrical charge) and electrons (which carry a negative electrical charge). An atom is the smallest unit of a pure substance or element that can exist and still retain the properties of the original substance or element.


 0 kommentar(er)
0 kommentar(er)
